The 2022 American Society of Hematology (ASH) Annual Meeting marked the 64th year in a row that hematology professionals gathered to discuss the latest research and updates in blood cancers and blood disorders. ASH is the world’s largest professional organizations made up of physicians and scientists with a keen interest in tackling blood diseases. Over 25,000 attendees participate in the annual ASH Meeting which aims to highlight advancements across both malignant and non-malignant hematology. Similar to last year’s meeting, ASH was hosted in a hybrid format, accommodating members from around the world to gather both virtually and in-person in New Orleans.
Each year, we look to honor and celebrate the Weill Cornell Medicine (WCM) Lymphoma Program team members whose new discoveries and research were selected for presentation at the ASH meeting. During the conference, we provided insights and perspectives via coverage on our Twitter feed, offering a deeper look into original research coming out of our basic science laboratories as well as translational and clinical research studies.
Leading up to the conference, Dr. John Leonard shared his #LeonardList: a yearly countdown on Twitter highlighting what he found to be the top 10 most impactful and important lymphoma research abstracts presented at the ASH annual meeting. For the fifth year in a row, a special CancerCast podcast episode provided listeners insight directly from Dr. Leonard regarding the “why” behind his #LeonardList selections, as well as 5 additional bonus podcast-only choices. His abstract selections aim to highlight research that is changing the treatment landscape for lymphoma patients, as well as other important issues as racial disparities and other factors in care. Listen to the teaser clip below for a sneak peek and tune into CancerCast for the full episode available on Apple Podcasts, Google Podcasts, Spotify, or online at Weill Cornell Medicine.
At this year’s ASH meeting, many of the notable presentations demonstrated scientific and treatment advancements that are leading the way for more targeted therapies and improved outcomes for patients. The WCM team shared research updates across many different forms of lymphoma.
Research involving Dr. Richard Furman investigated a novel treatment for people with lymphoma, including patients with mantle cell lymphoma (MCL), diffuse large B-cell lymphoma (DLBCL), and Richter’s transformation (RT). The study found that the treatment was tolerable and efficacious in well-treated and refractory patients, a population that often is less likely to respond to therapies. Dr. Furman breaks down the findings below.

The Weill Cornell Lymphoma Program is proud to partner with the Lymphoma Epidemiological Outcomes (LEO) Cohort, representing the largest, most diverse group of lymphoma patients studied to-date. At ASH 2022, our team was involved with many research updates involving this cohort. Our program chief, Dr. Peter Martin, shared his sentiments of gratitude, and our recent blog post details this LEO Cohort research.

Below is a breakdown of some of the additional great lymphoma work that Weill Cornell physicians and researchers shared throughout the ASH 2022 meeting.
Mantle Cell Lymphoma (MCL)
Dr. Jia Ruan presented data from an investigator-initiated multi-center study evaluating a triplet-combination therapy for mantle cell lymphoma (MCL) patients. In this phase II trial, the team added acalabrutinib to the doublet lenalidomide and rituximab treatment regimen with encouraging results. The study also explored minimal residual disease status as a biomarker to adjust treatment intensity and duration.

Diffuse Large B-Cell Lymphoma (DLBCL)
Dr. Mohammad Alhomoud, a current Weill Cornell Medicine and NewYork-Presbyterian Hospital Hematology & Medical Oncology fellow, participated in this year’s ASH conference. Below, he breaks down this research investigating the impact of low-dose radiation in combination with CAR T-cell therapy for diffuse large B-cell lymphoma (DLBCL).

Dr. Danny Luan presented at ASH 2022 on research he conducted under the mentorship of Weill Cornell Lymphoma Program Chief, Dr. Peter Martin, and other team members. Dr. Luan completed medical school at Weill Cornell and is now in his first-year residency at Weill Cornell and NewYork-Presbyterian Hospital. He reported on a new predictive model for DLBCL outcomes after patients complete immunochemotherapy.

Dr. Nicolás Di Siervi – a postdoctoral associate in Dr. Leandro Cerchietti’s lab –presented an oral abstract on a novel target for aggressive B-cell lymphomas. This research looked deeper into the tumor microenvironment to identify vulnerabilities that could be targeted for future lymphoma therapies.

T-Cell Lymphoma
Dr. Jia Ruan was selected to present research at this year’s ASH conference regarding how certain care factors affect clinical outcomes for peripheral T-cell lymphoma (PTCL) patients. Specifically, Dr. Ruan’s research dove into data from the Lymphoma Epidemiological Outcomes (LEO) and National Cancer Institute (NCI) supported Molecular Epidemiology Resource (MER) cohorts.

Chronic Lymphocytic Leukemia (CLL)
Dr. Richard Furman shared insights into results from a phase I/II clinical trial of acalabrutinib in relapsed and refractory chronic lymphocytic leukemia (CLL) patients. This research provided the longest follow-up dataset to-date for this subset of CLL patients. Dr. Furman explains the significance of this research below.

Dr. John Allan presented an oral abstract based on the CAPTIVATE study highlighting four year follow-up data from the minimal residual disease (MRD) study cohort of patients with chronic lymphocytic leukemia (CLL).

Finally, in addition to the important research that our team was involved with at this year’s ASH meeting, we had a few thought leaders selected to participate in specialized programming. Dr. John Leonard Chaired a Friday Satellite Symposium session in which Dr. John Allan was a panel speaker titled “Aggressive B-Cell Lymphomas: Experts Discuss New Care Standards and Evolving Treatment Strategies.” Alongside fellow lymphoma experts, Drs. Leonard and Allan helped educate attendees on innovative care strategies for aggressive B-cell lymphomas, including diffuse large B-cell lymphoma and mantle cell lymphoma.
Dr. Jia Ruan presented on “Fixing the Target on Aggressive Lymphoma: Insights on the Next Phase of Integrating Targeted Agents into MCL and DLBCL Management.” This CME program discussed the use of targeted agents, including new evidence with BTK inhibitors in MCL and DLBCL indicating that these therapies may lead to better treatment responses when added to established therapeutic platforms or when used in disease subtypes defined by specific molecular features.
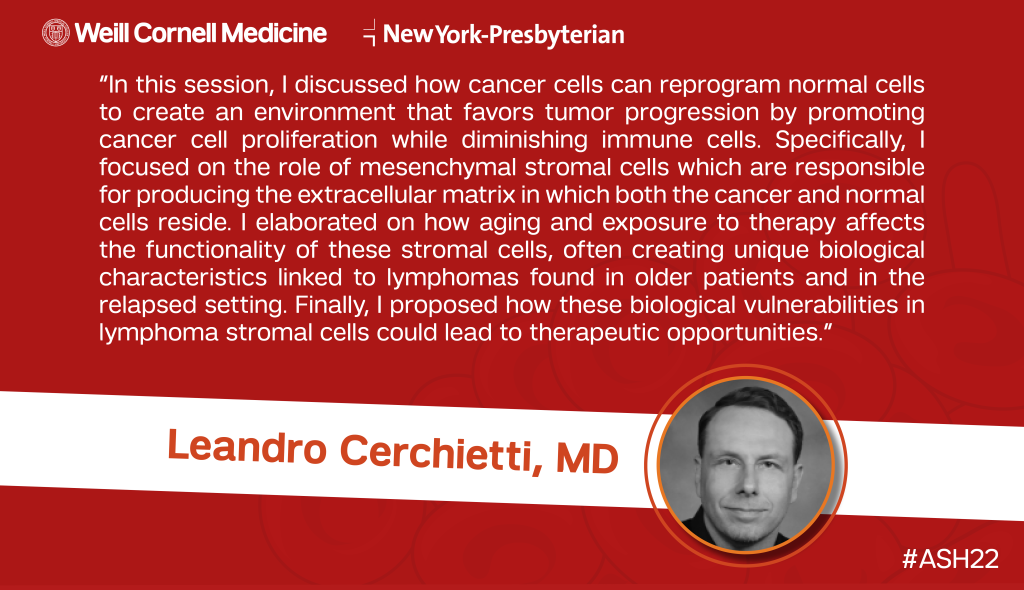
Dr. Leandro Cerchietti also spoke at a Scientific Program on Stromal Cells in Lymphoma, highlighting the role of these specialized cells in the lymphoma tumor microenvironment. Dr. Cerchietti discussed how leveraging certain biological vulnerabilities may lead to future therapies.
The Weill Cornell Medicine Lymphoma Program remains committed to advancing the overall understanding of lymphoma biology, improving clinical outcomes, and enhancing the quality of life for all those affected by the disease. While we are wrapping up coverage of our involvement at the 2022 ASH meeting, the WCM Lymphoma Program physicians and scientists continue to conduct research each and every day to advance the field.













 “The synergistic anti-lymphoma activity mediated by the combination of tazemetostat and venetoclax is quite promising,” says Dr. Roth. “This combination therapy is anticipated to be especially effective as precision therapy for DLBCL patients with EZH2 mutation and BCL2 alteration.”
“The synergistic anti-lymphoma activity mediated by the combination of tazemetostat and venetoclax is quite promising,” says Dr. Roth. “This combination therapy is anticipated to be especially effective as precision therapy for DLBCL patients with EZH2 mutation and BCL2 alteration.”